Volume 23, Issue 4 (October & November 2020)
J Arak Uni Med Sci 2020, 23(4): 570-579 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sheikhan M, Kordi M R, Rajabi H. Effect of a Lower Limb Restless Period on Expression of Mir-1 and Mir-206 Neural Muscle Genes in Endurance Training Rats. J Arak Uni Med Sci 2020; 23 (4) :570-579
URL: http://jams.arakmu.ac.ir/article-1-6120-en.html
URL: http://jams.arakmu.ac.ir/article-1-6120-en.html
1- Department of Sports Physiology, Faculty of Physical Education and Sport Sciences, Kish Campus, University of Tehran, Kish, Iran.
2- Department of Sports Physiology, Faculty of Physical Education and Sport Sciences, University of Tehran, Tehran, Iran. ,mrkordi@ut.ac.ir
3- Department of Sport Physiology, Faculty of Physical Education and Sports Sciences, Kharazmi University, Tehran, Iran.
2- Department of Sports Physiology, Faculty of Physical Education and Sport Sciences, University of Tehran, Tehran, Iran. ,
3- Department of Sport Physiology, Faculty of Physical Education and Sports Sciences, Kharazmi University, Tehran, Iran.
Full-Text [PDF 4407 kb]
(1241 Downloads)
| Abstract (HTML) (3507 Views)
Full-Text: (1743 Views)
1. Introduction
uscle atrophy is caused by the aging, inactive lifestyle, suspension, and a variety of pathological conditions [1]. Exercise can cause hypertrophy by inhibiting atrophic factors such as FOXO by developing the AKT pathway [3]. FOXO is inhibited by AKT and PGC-1α and decreased expression of PGC-1α has also been observed in inactivity [4]. Little information is known about the role of miRNAs and their effects in the sedentary period after a training period [11]. miR-1 and miR-206 because they play an important role in increasing and inhibiting and on the other hand miR-1 is directly regulated by FOXO3a and inhibits IGF-1 and in contrast miR-206 through MyoD degradation was able to reduce FOXO3a The result is a lack of reduction in IGF-1 and ultimately maintenance of protein synthesis in inactivity [13]. This study aimed to investigate the expression of miR-1, miR-206, IGF-1, and FoxO3a genes following a short period of inactivity in trained and untrained rats.
2. Materials and Methods
The present study was fundamental and experimental. A total of 18 male Sprague-Dawley rats at the age of eight weeks with an average weight of 200±20 g were randomly divided into control groups (n=6) and endurance training groups (n=12). The rats in the endurance training group performed endurance training for 6 weeks and five days a week. Training started at 17.5 meters per minute for 15 minutes and reached 30 meters per minute for 60 minutes. The control group experienced walking on a treadmill at a speed of five meters per minute for 15 minutes [15]. The rats were anesthetized 48 hours after the last training session.
Both legs were then fixed at the hip, knee (in extension), and ankle (in plantar flexion) [16] for seven days. Immobilization of the lower extremities and the ability to freely consume water and food was confirmed by observation. In the end, all rats were anesthetized and soleus muscle was extracted and the expression of the desired genes was measured by the RT-PCR method and the ratio of the target gene to reference gene was calculated using CT-2 formula. To determine the differences, the one-way ANOVA method and Tukey post hoc test were used at a significant level (α≥0.05).
Both legs were then fixed at the hip, knee (in extension), and ankle (in plantar flexion) [16] for seven days. Immobilization of the lower extremities and the ability to freely consume water and food was confirmed by observation. In the end, all rats were anesthetized and soleus muscle was extracted and the expression of the desired genes was measured by the RT-PCR method and the ratio of the target gene to reference gene was calculated using CT-2 formula. To determine the differences, the one-way ANOVA method and Tukey post hoc test were used at a significant level (α≥0.05).
3. Results
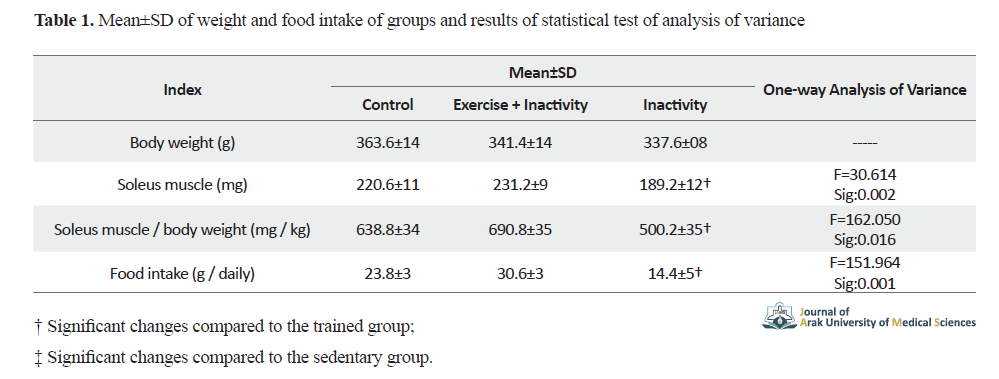
Soleus muscle weight The ratio of horseshoe muscle weight to body weight and food intake of the exercise group compared to the sedentary-exercise + sedentary group were statistically significant (Table 1).
Increased expression of miR-1 (P=0.012), FOXO3a (P=0.001) genes and decreased expression of miR-206 (P=0.007) and IGF-1 (P=0.003) in the exercise+inactivity group and the inactivity group compared to the control group, these differences were statistically significant. Also, the in-group results showed a non-significant increase in miR-1 gene expression (P=0.068) in the inactive group compared to the exercise+inactivity group, but an increase in FOXO3a (P=0.004) in the inactive group compared to the exercise + group. Inactivity was significant. Also, the inactive group showed a significant decrease in the expression of miR-206 (P=0.030) and IGF-1 (P=0.002) genes compared to the exercise + inactivity group (Figure 1).
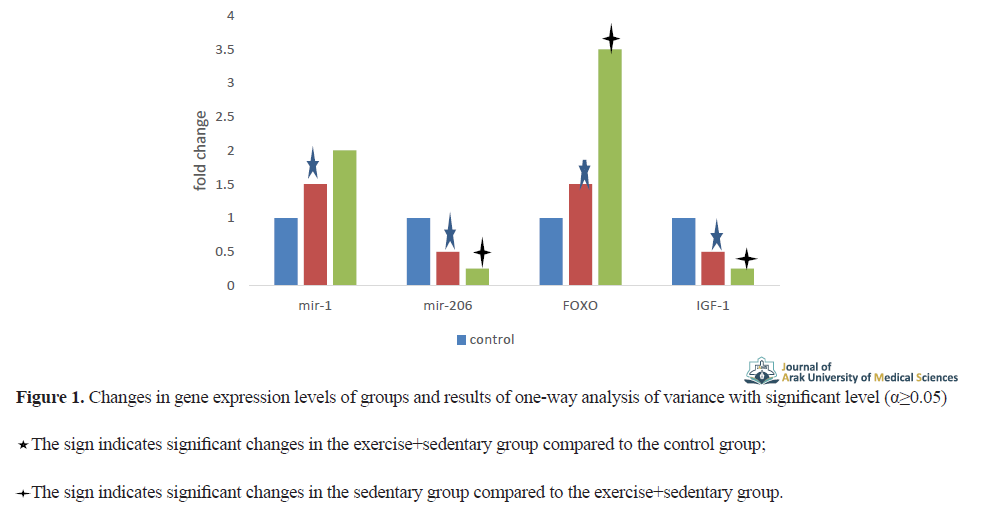

Increased expression of miR-1 (P=0.012), FOXO3a (P=0.001) genes and decreased expression of miR-206 (P=0.007) and IGF-1 (P=0.003) in the exercise+inactivity group and the inactivity group compared to the control group, these differences were statistically significant. Also, the in-group results showed a non-significant increase in miR-1 gene expression (P=0.068) in the inactive group compared to the exercise+inactivity group, but an increase in FOXO3a (P=0.004) in the inactive group compared to the exercise + group. Inactivity was significant. Also, the inactive group showed a significant decrease in the expression of miR-206 (P=0.030) and IGF-1 (P=0.002) genes compared to the exercise + inactivity group (Figure 1).

4. Discussion and Conclusion
The most important findings of this study were the increase in the expression of atrophy stimulus genes (miR-1 and FOXO3a) and the decrease in the expression of muscle hypertrophy stimulus genes (miR-206 and IGF-1) in the exercise+inactivity group and the inactivity group compared to the control group, which confirms the principle of training reversibility. Endurance exercise activities with increased aerobic performance and development of antioxidant capacity create favorable conditions for hypertrophy.
By decreasing reactive oxygen species and increasing PGC-1α expression, the FOXO3a-associated atrophy signal pathways associated with miR-1 are reduced. Exercise appears to increase IGF-1, which is associated with miR-206. With inactivity, the first thing that happens in the muscles is a decrease in aerobic power, which has been shown to decrease PGC-1α expression. This reduction reduces the intracellular content of muscle, especially intracellular water, which in turn reduces antioxidant defenses, and ultimately allows atrophic pathways to grow due to the absence of hypertrophic agents.
Despite the protective effects of exercise activities against atrophy due to inactivity, the effect of complete inactivity will be greater due to high levels of fitness. This process is achieved by reducing the stimulants of hypertrophy and increasing the inhibitory factors by reducing the antioxidant defense and will play a very important role in the development of growth hormones. In addition, very important cellular markers called miR-206 are reduced, which causes the atrophy process to continue. In spite of the importance of preventing this regressive process and reducing ideal bodily function, there is still no clear way to deal with this inactive condition and the need for further studies and training (nutrition training) and nutritional interventions (polyphenols such as quercetin and curcumin) is felt.
By decreasing reactive oxygen species and increasing PGC-1α expression, the FOXO3a-associated atrophy signal pathways associated with miR-1 are reduced. Exercise appears to increase IGF-1, which is associated with miR-206. With inactivity, the first thing that happens in the muscles is a decrease in aerobic power, which has been shown to decrease PGC-1α expression. This reduction reduces the intracellular content of muscle, especially intracellular water, which in turn reduces antioxidant defenses, and ultimately allows atrophic pathways to grow due to the absence of hypertrophic agents.
Despite the protective effects of exercise activities against atrophy due to inactivity, the effect of complete inactivity will be greater due to high levels of fitness. This process is achieved by reducing the stimulants of hypertrophy and increasing the inhibitory factors by reducing the antioxidant defense and will play a very important role in the development of growth hormones. In addition, very important cellular markers called miR-206 are reduced, which causes the atrophy process to continue. In spite of the importance of preventing this regressive process and reducing ideal bodily function, there is still no clear way to deal with this inactive condition and the need for further studies and training (nutrition training) and nutritional interventions (polyphenols such as quercetin and curcumin) is felt.
Ethical Considerations
Compliance with ethical guidelines
This paper ethically approved by the research ethics committee of Tehran University of Medical Sciences (Code: IR.SUMS.REC.2017.S444).
Funding
The paper was extracted from the PhD. dissertation of the first author, the Department of Sports Physiology, Faculty of Physical Education and Sport Sciences, Kish Campus, University of Tehran.
Authors' contributions
All authors contributed in preparing this article.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors would like to thank dr. Mohammad Reza Kordi and dr. Hamid Rajabi.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









