BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://jams.arakmu.ac.ir/article-1-6202-en.html
2- Department of Biology, Faculty of Sciences, University of Mohaghegh Ardabili, Ardabil, Iran. ,
Introduction
Polycystic Ovary Syndrome (PCOS) is associated with insulin resistance, elevated serum androgen levels, and an increase in the ratio of Luteinizing Hormone (LH) to Follicle-Stimulating Hormone (FSH) [1, 2]. Adiponectin is synthesized in adipose tissue, hypothalamus, and gonads [3, 4]. Deficiency in adiponectin production leads to insulin resistance and disruption in lipid and glucose metabolisms [7, 8]. Serum adiponectin levels in PCOS women are lower than in healthy individuals [8, 10]. L-dopa is a precursor to the neurotransmitters dopamine, epinephrine and norepinephrine [14]. Dopamine and L-dopa inhibit Hypothalamic-Pituitary-Gonadal (HPG) axis activity [15, 16]. PCOS is associated with decreased dopamine release [19]. This study aimed to examine the effects of L-dopa and dopamine receptor antagonists (SCH 23390 as D1 receptor and sulperide as D2 receptor) on LH secretion and relative expression of adiponectin gene in the hypothalamus and ovaries of rats with PCOS induced by
Materials and Methods
To perform this study, 20 Wistar female rats weighing 180-220 g were used. In order to induce PCOS, the animals received intramuscular injection of Estradiol Valerate (EV) in the estrous stage. 15 PCOS rats were divided into three groups of saline, L-dopa (100 mg/kg), and L-dopa + sulpiride + SCH 23390 (100 mg/kg L-dopa + 10mg/kg sulpiride + 1mg/kg SCH 23390 hydrochloride), and 5 healthy rats received saline as negative control group. In groups receiving antagonist and L-dopa, antagonists were injected 10 minutes before L-dopa injection. The hypothalamus and ovarian samples were isolated and stored at -80°C until RNA extraction. The average serum LH concentration was measured using Radioimmunoassay (RIA). The mean relative expression of adiponectin gene in the ovaries and hypothalamus was calculated using real-time PCR assay and delta-delta CT method ( 2-∆∆Ct formula). The data obtained from this formula were analyzed in SPSS V. 16 software using one-way ANOVA test and the mean data were compared by using Tukey’s post-hoc test. The results were presented as Mean±SD, considering the significance level of P≤0.05.
Results
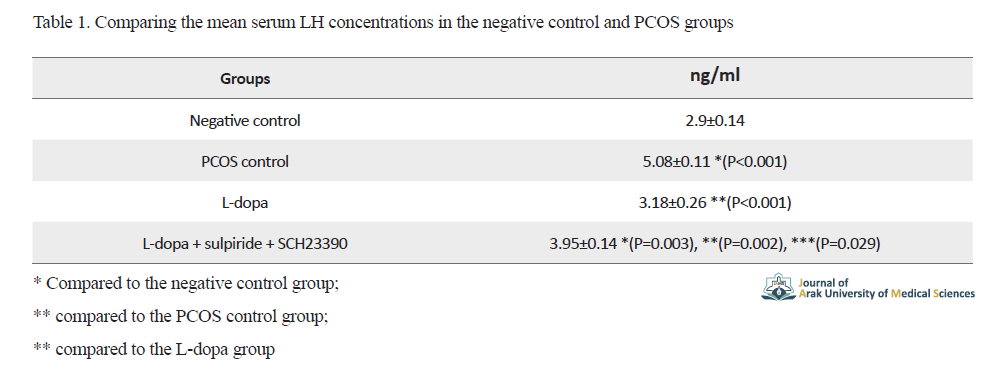
The results of comparing the mean serum LH concentrations in the negative control and PCOS groups are shown in Table 1.
The mean relative expression of adiponectin gene in the ovaries and hypothalamus of the PCOS control group showed a significant decrease compared to the negative control group (P=0.001 for ovaries and P=0.015 for hypothalamus) (Figure 1).
For the PCOS group received L-dopa only, the mean relative expression of adiponectin gene in the ovaries and hypothalamus increased non-significantly (P=0.924) and significantly (P<0.001), respectively (Figure 1). For the PCOS group received L-dopa, sulpiride, and SCH 23390 hydrochloride simultaneously, the mean relative expression of adiponectin gene in the ovaries and hypothalamus decreased non-significantly (P=0.948) and significantly (P=0.025), respectively (Figure 1).
Discussion
The results of the present study showed that in PCOS rats, serum LH concentrations increased significantly compared to the healthy rats, while the mean relative expression of adiponectin gene in the ovaries and hypothalamus of PCOS rats reduced significantly compared to the healthy rats. The results are consistent with previous research on the extent of adiponectin secretion in humans and rodents with PCOS. Previous studies have shown that the serum adiponectin level is reduced in women with PCOS compared to healthy peers, and PCOS obese women have lower serum levels than non-PICOS obese women [8, 22]. Decreased adiponectin levels in PCOS women may be due to increased production of androgens caused by reduced inhibitory effects of adiponectin on theca cells [23], because hyperandrogenism and obesity have been shown to play an important role in reducing plasma levels of adiponectin and causing insulin resistance, which is a major feature of PCOS [24, 25].
L-dopa exerted inhibitory effects on LH secretion and stimulatory effects on adiponectin gene expression in the hypothalamus of PCOS rats; however, it had no stimulatory effects on adiponectin gene expression in the ovaries of PCOS rats. This is consistent with the results of an in-vitro study where researchers examined the effects of dopamine on adipocyte cells incubated in culture, and reported the stimulatory effects of dopamine on adiponectin secretion from these cells [22]. Dopamine receptor antagonists including SCH23390 hydrochloride and sulpiride blocked the inhibitory effects of L-dopa on the LH secretion and its stimulatory effects on the relative expression of the adiponectin gene in the hypothalamus of PCOS rats. Increasing the activity of dopaminergic neurons may be effective in controlling endocrine disorders caused by decreased adiponectin secretion in PCOS patients.
Ethical Considerations
Compliance with ethical guidelines
This study ethically approved in ethics committee of University of Mohaghegh Ardabili (Code: 95-125-1).
Funding
The present paper was extracted from the MSc thesis of the first author, Department of Biology, Faculty of Sciences, University of Mohaghegh Ardabili.
Authors' contributions
All authors contributed in preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Deputy of Research and Technology of the Mohaghegh Ardabili University for their financial and non-financial supports. Also thank to Dr. Homayoun Khaz'ali from Shahid Beheshti University for providing the instruments.
References
1.Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2017; 40(1):1-8. [DOI:10.1007/s40618-016-0523-8] [PMID] [PMCID]
2.Adgi Z, Talaei A, Mohamadi Kelishadi M. [The evaluation of the relationship between hirsutism and insulin resistance in patients with PCOS and idiopathic hirsutism (Persian)]. J Arak Univ Med Sci. 2011; 14(2):51-7. http://jams.arakmu.ac.ir/article-1-953-en.html
3.Dobrzyn K, Smolinska N, Kiezun M, Szeszko k, Rytelewska E, Kisielewska K, et al. Adiponectin: A new regulator of female reproductive system. Int J Endocrinol. 2018; 2018:7965071. [DOI:10.1155/2018/7965071] [PMID] [PMCID]
4.Davoodi B, Zilaei Bouri Sh, Ahangarpor A, Zilaei Bouri M. [Effects of two different physical exercises on plasma levels of adiponectin and resistin in obese and overweight young girls (Persian)]. J Arak Univ Med Sci. 2014; 17(4):27-37. http://jams.arakmu.ac.ir/article-1-2206-en.html
5.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007; 148(1):401-10. [DOI:10.1210/en.2006-1019] [PMID]
6.Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014; 15(2):149-56. [DOI:10.1007/s11154-013-9283-3] [PMID] [PMCID]
7.Groth SW. Adiponectin and polycystic ovary syndrome. Biol Res Nurs. 2010; 12(1):62-72. [DOI:10.1177/1099800410371824] [PMID] [PMCID]
8.Michalakis KG, Segars JH. The role of adiponectin in reproduction: From polycystic ovary syndrome to assisted reproduction. Fertil Steril. 2010; 94(6):1949-57. [DOI:10.1016/j.fertnstert.2010.05.010] [PMID] [PMCID]
9.Cheng XB, Wen JP, Yang J, Yang Y, Ning G, Li XY. GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine. 2011; 39(1):6-12. [DOI:10.1007/s12020-010-9375-8] [PMID]
10.Parillo F, Maranesi M, Mignini F, Marinelli L, Di Stefano A, Boiti C, et al. Evidence for a dopamine intrinsic direct role in the regulation of the ovary reproductive function: In vitro study on rabbit corpora lutea. PLoS One. 2014; 9(8):e104797. [DOI:10.1371/journal.pone.0104797] [PMID] [PMCID]
11.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007; 30(5):194-202. [DOI:10.1016/j.tins.2007.03.006] [PMID]
12.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000; 24(1):125-32. [DOI:10.1016/S0149-7634(99)00063-9]
13.Fontaine R, Affaticati P, Yamamoto K, Jolly C, Bureau C, Baloche S, et al. Dopamine inhibits reproduction in female zebrafish (Danio rerio) via three pituitary D2 receptor subtypes. Endocrinology. 2013; 154(2):807-18. [DOI:10.1210/en.2012-1759] [PMID]
14.Zarabian M, Salehipour F, Ostad SN. The study of dose-response mitogenic effect of L-dopa on the human periodontal ligament fibroblasts cell. Acta Med Iran. 2004; 42(5):363-6. https://acta.tums.ac.ir/index.php/acta/article/view/2752
15.Liu X, Herbison AE. Dopamine regulation of gonadotropin-releasing hormone neuron excitability in male and female mice. Endocrinology. 2013; 154(1):340-50. [DOI:10.1210/en.2012-1602] [PMID]
16.Venegas-Meneses B, Padilla JF, Juárez CE, Morán JL, Morán C, Rosas-Murrieta NH, et al. Effects of ovarian dopaminergic receptors on ovulation. Endocrine. 2015; 50(3):783-96. [DOI:10.1007/s12020-015-0636-4] [PMID]
17.Chaudhari N, Dawalbhakta M, Nampoothiri L. GnRH dysregulation in Polycystic Ovarian Syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod Biol Endocrinol. 2018; 16(1):37. [DOI:10.1186/s12958-018-0354-x] [PMID] [PMCID]
18.Ayano G. Dopamine: Receptors, functions, synthesis, pathways, locations and mental disorders: Review of literatures. J Ment Disord Treat. 2016; 2(2):1000120. [DOI:10.4172/2471-271X.1000120]
19.Gómez R, Ferrero H, Delgado-Rosas F, Gaytan M, Morales C, Zimmermann RC, et al. Evidences for the existence of a low dopaminergic tone in polycystic ovarian syndrome: Implications for OHSS development and treatment. J Clin Endocrinol Metab. 2011; 96(8):2484-92. [DOI:10.1210/jc.2011-0075] [PMID]
20.Andersson K, Fuxe K, Eneroth P, Härfstrand A, Agnati LF. Involvement of D1 dopamine receptors in the nicotine-induced neuro-endocrine effects and depletion of diencephalic catecholamine stores in the male rat. Neuroendocrinology. 1988; 48(2):188-200. [DOI:10.1159/000125007] [PMID]
21.Grierson JP, James MD, Pearson JR, Wilson CA. The effect of selective D1 and D2 dopaminergic agents on sexual receptivity in the female rat. Neuropharmacology. 1988; 27(2):181-9. [DOI:10.1016/0028-3908(88)90169-4]
22.Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J, et al. Dopamine receptors in human adipocytes: Expression and functions. PloS One. 2011; 6(9):e25537. [DOI:10.1371/journal.pone.0025537] [PMID] [PMCID]
23.Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008; 284(1-2):38-45. [DOI:10.1016/j.mce.2008.01.007] [PMID]
24.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Álvarez-Blasco F, Sanchón R, Luque-Ramírez M, et al. Adiponectin and resistin in PCOS: A clinical, biochemical and molecular genetic study. Hum Reprod. 2006; 21(9):2257-65. [DOI:10.1093/humrep/del146] [PMID]
25.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002; 51(9):2734-41. [DOI:10.2337/diabetes.51.9.2734] [PMID]
26.Nilsson L, Binart N, Bohlooly-Y M, Bramnert M, Egecioglu E, Kindblom J, et al. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun. 2005; 331(4):1120-6. [DOI:10.1016/j.bbrc.2005.04.026] [PMID]
27.Daimon M, Kamba A, Murakami H, Mizushiri S, Osonoi Sh, Yamaichi M, et al. Association between serum prolactin levels and insulin resistance in non-diabetic men. PLoS One. 2017; 12(4):e0175204. [DOI:10.1371/journal.pone.0175204] [PMID] [PMCID]
28.Xu A, Chan KW, Hoo RLC, Wang Y, Tan KCB, Zhang J, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005; 280(18):18073-80. [DOI:10.1074/jbc.M414231200] [PMID]
29.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005; 26(1):85-92. [DOI:10.1002/j.1939-4640.2005.tb02876.x]
30.Giahi L, Djazayery A, Rahimy A, Rahmany M, Larijani B. Serum level of adiponectin and its association with insulin sensitivity in overweight diabetic and non-diabetic Iranian men. Iran J Public Health. 2008; 37(2):88-92. https://ijph.tums.ac.ir/index.php/ijph/article/view/2060
31.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001; 7(8):947-53. [DOI:10.1038/90992] [PMID]
Received: 2019/12/3 | Accepted: 2020/02/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |