Volume 22, Issue 5 (11-2019)
J Arak Uni Med Sci 2019, 22(5): 18-31 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hajihashemi S, Rajabi R, Ghiasabadi Farahani A. The Oral Post-Treatment Effect of Hydroethanolic Extract of Origanum Vulgare on Acute Kidney Injury Caused by Gentamicin in Rats. J Arak Uni Med Sci 2019; 22 (5) :18-31
URL: http://jams.arakmu.ac.ir/article-1-6131-en.html
URL: http://jams.arakmu.ac.ir/article-1-6131-en.html
1- Department of Physiology, School of Medicine, Arak University of Medical Sciences, Arak, Iran. , s.hajihashemi@gmail.com
2- Department of Physiology, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
2- Department of Physiology, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
Full-Text [PDF 6536 kb]
(1403 Downloads)
| Abstract (HTML) (3587 Views)
Discussion
The results showed that Origanum vulgare ethanol extract after oral treatment reduces the effects of gentamicin induced nephrotoxicity. The results showed that gentamicin increased plasma creatinine and urea levels, decreased creatinine and urea clearance and consequently decreased urinary excretion. This change indicated impair in the kidney function and induce renal toxicity. These changes in renal function may be due to a decrease in Glomerular Filtration Rate (GFR) and cell necrosis as well as tubular cell loss [17].
Similar to previous studies, gentamicin induced tubular cell necrosis, tubular cell shedding, formation of protein molds in the lumen, vacuolization of the tubular cells, decreased red blood cell count in the glomerulus, and increased Bowman capsule space [15]. Origanum vulgare ethanol extract can decrease the amount of tubular cell necrosis, decrease in tubular cells proliferation, formation of protein casts in the lumen, vacuolation of the tubular cells, and decrease of Bowman capsule space on the tissue changes caused by gentamicin.
Gentamicin produces oxidative stress by generating ROS. The balance between antioxidant ability and production of ROS is impaired. ROS resulting from oxidative stress, including superoxide anions and hydroxyl radicals, increases cell damage anddeath [19].
Gentamicin induces glomerular mesangial cells contraction by producing ROS and decreases GFR by decreasing filtration coefficient (Kf) [22]. With the decrease in GFR, creatinine clearance also decreased, which increased the creatinine and urea in the blood. The use of antioxidants by reducing free radicals prevents contraction of mesangial cells and consequently decreases GFR and consequently increases creatinine clearance and decreases plasma urea and creatinine concentration.
Ethical Considerations
Compliance with ethical guidelines
This study obtained its ethical approval form the Research Ethics Committee of Arak University of Medical Sciences (Code: 90-106-8). All tests on animals were approved by the ethics committee for laboratory animals of this university.
Funding
This study extracted from a research proposal approved by the Arak University of Medical Sciences. We received financial support from the Deputy for Research and Technology of this university.
Authors' contributions
Conceptualization, methodology, validation, and formal analysis: Saeed Hajihashemi; Investigation, resources, analysis and initial draft preparation: Razieh Rajabi and Atefeh Ghiasabadi Farahani.
Conflicts of interest
The authors declare no conflict of interest
Acknowledgements
The authors would like to thank the Deputy for Research and Technology of Arak University of Medical Sciences for their support.
References
Full-Text: (3031 Views)
Introduction
Acute Kidney Injury (AKI) is a side effect of aminoglycoside antibiotics, including gentamicin, which is used to treat infections associated with gram-negative bacteria. Gentamicin treatment causes about 10%-15% of acute renal injury [2 ,1]. Despite the adverse effects of gentamicin such as nephrotoxicity and ototoxicity due to the rapid antibacterial effect and wide spectrum of activity and chemical structure stability and low cost, gentamicin is still being used [3]. The mechanisms of gentamicin induced nephrotoxicity are not completely known.
Previous research showed gentamicin increase the production of Reactive Oxygen Species (ROS) such as super oxide anions. Gentamicin can damage different types of kidney cells such as tubular epithelial cell and glomerular as well as renal artery. In glomeruli, gentamicin can decrease glomerular filtration coefficient (Kf) and decrease glomerular filtration by decrease glomerular mesangial cells.
Previous study has shown the anti-inflammatory and antioxidant effects of flavonoids found in Origanum vulgare. Flavonoids have inhibitory effects on cyclooxygenases or the 5-lipoxygenase pathway in arachidonic acid metabolism. Hydroethanolic Origanum vulgare extract is rich in phenolic antioxidants increasing antioxidant power, reduce oxygen-free radicals known as ROS including superoxide and hydroxyl radicals and peroxides such as hydrogen peroxide [7 ,6]. Previous studies have shown that ROS can cause tissue damage, but flavonoids in the Origanum vulgare extract, such as other plants such as rosemary, rosacanina, etc., have protective effects by reducing ROS in inflammatory processes [9 ,8].
Nephrotoxicity of gentamicin is induced by producing of free radicals and causing oxidative stress and reducing the power of the antioxidant defense mechanism. Therefore, due to the anti-inflammatory and antioxidant properties of the compounds in the Origanum vulgare extract, we investigated the effects of the Origanum vulgare extract on disorders caused by acute renal injury induced by gentamicin in male rats.
Materials and Methods
This experimental study was performed on 32 adult Wistar rats. Hydroethanolic extract were obtained from the leaves of Origanum vulgare by rotatory evaporator. Experimental group including:
1. Control group, without receiving gentamicin and Origanum vulgare extract; 2. Gentamicin group, (100 mg/kg/day) with intraperitoneally injection of gentamicin for 8 days and 4 ml/kg distilled water by gavage for two days; 3. Origanum vulgare extract group, intraperitoneally injection of normal saline for 8 days and Origanum vulgare extract (40 mg/kg) by gavage for two days; 4. Gentamicin and Origanum vulgare extract post treatment group, intraperitoneally injection of gentamicin (100 mg/kg/day) for 8 days and Origanum vulgare extract (40 mg/kg) by gavage for two days.
On the eleven day of the post-treatment protocol the animals were placed in metabolic cages and their urine was collected. The systolic blood pressure was measured from caudal artery using tail cuff of Power Lab device (AD Instruments, Australia). Blood samples were obtained from the abdominal aorta by a cold heparinized syringe. After separation of plasma, the concentration of urea, creatinine, sodium, potassium and osmolarity were measured in plasma and urine samples. Creatinine and Blood Urea Nitrogen (BUN), concentrations of sodium and potassium (K) were measured in plasma and urine samples. Creatinine Clearance (CCr) and absolute and relative sodium and potassium excretion values were also calculated. Both kidneys was separated and left kidneys were fixed in 10% formalin for histology study. Right kidney was used for MDA (Malondialdehyde) and FRAP (ferric reducing antioxidant power) experiment.
Results
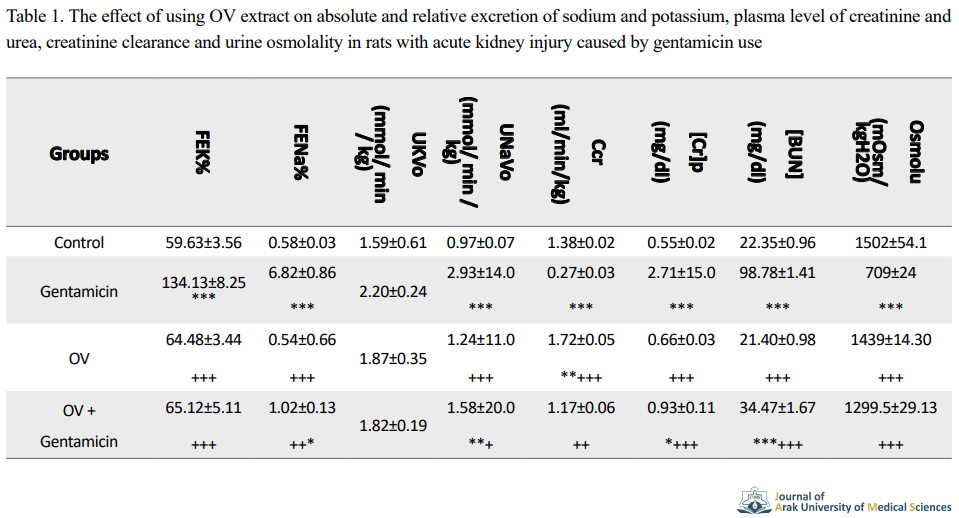
Clearance of creatinine was significantly decreased in the gentamicin group compared to the control group. A significant increase in creatinine clearance was observed in gentamicin-treated rats along with Origanum vulgare extract compared to the gentamicin-treated group.
Relative sodium excretion was significantly decreased in gentamicin-treated rats along with Origanum vulgare extract compared to gentamicin-treated rats. Treated with Origanum vulgare extract was significantly decreased relative potassium excretion in compared to the gentamicin group. In gentamicin-treated rats treated with Origanum vulgare extract the amount of absolute sodium excretion showed a significant decrease in compared to the gentamicin group.
Urinary sodium and potassium excretion were significantly increased in the gentamicin group compared to the control group. There was a significant decrease in urinary sodium and potassium excretion in gentamicin-treated rats along with Origanum vulgare ethanol extract compared to gentamicin group. Gentamicin significantly increased urinary creatinine concentration compared to the control group. Gentamicin-treated mice treated with ethanolic Origanum vulgare extract showed a significant decrease in urinary creatinine concentration compared to gentamicin group.
Gentamicin significantly decreased urinary urea excretion compared to the control group. Gentamicin-treated mice treated with ethanolic extract of Origanum vulgare showed a significant increase in urinary urea excretion compared to gentamicin group. Gentamicin-treated rats treated with ethanolic extract of Origanum vulgare showed significantly increased urinary osmolality compared to the gentamicin-treated group.
Gentamicin significantly increased plasma creatinine and blood urea nitrogen concentration compared to the control group. Plasma creatinine and blood urea nitrogen concentration in gentamicin-treated rats along with Origanum vulgare extract significantly decreased compared to gentamicin group (Table 1).
Acute Kidney Injury (AKI) is a side effect of aminoglycoside antibiotics, including gentamicin, which is used to treat infections associated with gram-negative bacteria. Gentamicin treatment causes about 10%-15% of acute renal injury [2 ,1]. Despite the adverse effects of gentamicin such as nephrotoxicity and ototoxicity due to the rapid antibacterial effect and wide spectrum of activity and chemical structure stability and low cost, gentamicin is still being used [3]. The mechanisms of gentamicin induced nephrotoxicity are not completely known.
Previous research showed gentamicin increase the production of Reactive Oxygen Species (ROS) such as super oxide anions. Gentamicin can damage different types of kidney cells such as tubular epithelial cell and glomerular as well as renal artery. In glomeruli, gentamicin can decrease glomerular filtration coefficient (Kf) and decrease glomerular filtration by decrease glomerular mesangial cells.
Previous study has shown the anti-inflammatory and antioxidant effects of flavonoids found in Origanum vulgare. Flavonoids have inhibitory effects on cyclooxygenases or the 5-lipoxygenase pathway in arachidonic acid metabolism. Hydroethanolic Origanum vulgare extract is rich in phenolic antioxidants increasing antioxidant power, reduce oxygen-free radicals known as ROS including superoxide and hydroxyl radicals and peroxides such as hydrogen peroxide [7 ,6]. Previous studies have shown that ROS can cause tissue damage, but flavonoids in the Origanum vulgare extract, such as other plants such as rosemary, rosacanina, etc., have protective effects by reducing ROS in inflammatory processes [9 ,8].
Nephrotoxicity of gentamicin is induced by producing of free radicals and causing oxidative stress and reducing the power of the antioxidant defense mechanism. Therefore, due to the anti-inflammatory and antioxidant properties of the compounds in the Origanum vulgare extract, we investigated the effects of the Origanum vulgare extract on disorders caused by acute renal injury induced by gentamicin in male rats.
Materials and Methods
This experimental study was performed on 32 adult Wistar rats. Hydroethanolic extract were obtained from the leaves of Origanum vulgare by rotatory evaporator. Experimental group including:
1. Control group, without receiving gentamicin and Origanum vulgare extract; 2. Gentamicin group, (100 mg/kg/day) with intraperitoneally injection of gentamicin for 8 days and 4 ml/kg distilled water by gavage for two days; 3. Origanum vulgare extract group, intraperitoneally injection of normal saline for 8 days and Origanum vulgare extract (40 mg/kg) by gavage for two days; 4. Gentamicin and Origanum vulgare extract post treatment group, intraperitoneally injection of gentamicin (100 mg/kg/day) for 8 days and Origanum vulgare extract (40 mg/kg) by gavage for two days.
On the eleven day of the post-treatment protocol the animals were placed in metabolic cages and their urine was collected. The systolic blood pressure was measured from caudal artery using tail cuff of Power Lab device (AD Instruments, Australia). Blood samples were obtained from the abdominal aorta by a cold heparinized syringe. After separation of plasma, the concentration of urea, creatinine, sodium, potassium and osmolarity were measured in plasma and urine samples. Creatinine and Blood Urea Nitrogen (BUN), concentrations of sodium and potassium (K) were measured in plasma and urine samples. Creatinine Clearance (CCr) and absolute and relative sodium and potassium excretion values were also calculated. Both kidneys was separated and left kidneys were fixed in 10% formalin for histology study. Right kidney was used for MDA (Malondialdehyde) and FRAP (ferric reducing antioxidant power) experiment.
Results
Clearance of creatinine was significantly decreased in the gentamicin group compared to the control group. A significant increase in creatinine clearance was observed in gentamicin-treated rats along with Origanum vulgare extract compared to the gentamicin-treated group.
Relative sodium excretion was significantly decreased in gentamicin-treated rats along with Origanum vulgare extract compared to gentamicin-treated rats. Treated with Origanum vulgare extract was significantly decreased relative potassium excretion in compared to the gentamicin group. In gentamicin-treated rats treated with Origanum vulgare extract the amount of absolute sodium excretion showed a significant decrease in compared to the gentamicin group.
Urinary sodium and potassium excretion were significantly increased in the gentamicin group compared to the control group. There was a significant decrease in urinary sodium and potassium excretion in gentamicin-treated rats along with Origanum vulgare ethanol extract compared to gentamicin group. Gentamicin significantly increased urinary creatinine concentration compared to the control group. Gentamicin-treated mice treated with ethanolic Origanum vulgare extract showed a significant decrease in urinary creatinine concentration compared to gentamicin group.
Gentamicin significantly decreased urinary urea excretion compared to the control group. Gentamicin-treated mice treated with ethanolic extract of Origanum vulgare showed a significant increase in urinary urea excretion compared to gentamicin group. Gentamicin-treated rats treated with ethanolic extract of Origanum vulgare showed significantly increased urinary osmolality compared to the gentamicin-treated group.
Gentamicin significantly increased plasma creatinine and blood urea nitrogen concentration compared to the control group. Plasma creatinine and blood urea nitrogen concentration in gentamicin-treated rats along with Origanum vulgare extract significantly decreased compared to gentamicin group (Table 1).
Treatment with Origanum vulgare extract significantly increased FRAP in kidney tissue compared to gentamicin group. In the gentamicin group, MDA level in kidney tissue was significantly increased compared to the control group. After treatment with Origanum vulgare extract, MDA 54% decreased compared to the gentamicin group (Figure 2). Histology studies showed that gentamicin causes severe damage to the kidney tissue. Microscopic examination showed that the Origanum vulgare extract significantly improved gentamicin induced tissue damage.
Discussion
The results showed that Origanum vulgare ethanol extract after oral treatment reduces the effects of gentamicin induced nephrotoxicity. The results showed that gentamicin increased plasma creatinine and urea levels, decreased creatinine and urea clearance and consequently decreased urinary excretion. This change indicated impair in the kidney function and induce renal toxicity. These changes in renal function may be due to a decrease in Glomerular Filtration Rate (GFR) and cell necrosis as well as tubular cell loss [17].
Similar to previous studies, gentamicin induced tubular cell necrosis, tubular cell shedding, formation of protein molds in the lumen, vacuolization of the tubular cells, decreased red blood cell count in the glomerulus, and increased Bowman capsule space [15]. Origanum vulgare ethanol extract can decrease the amount of tubular cell necrosis, decrease in tubular cells proliferation, formation of protein casts in the lumen, vacuolation of the tubular cells, and decrease of Bowman capsule space on the tissue changes caused by gentamicin.
Gentamicin produces oxidative stress by generating ROS. The balance between antioxidant ability and production of ROS is impaired. ROS resulting from oxidative stress, including superoxide anions and hydroxyl radicals, increases cell damage anddeath [19].
Gentamicin induces glomerular mesangial cells contraction by producing ROS and decreases GFR by decreasing filtration coefficient (Kf) [22]. With the decrease in GFR, creatinine clearance also decreased, which increased the creatinine and urea in the blood. The use of antioxidants by reducing free radicals prevents contraction of mesangial cells and consequently decreases GFR and consequently increases creatinine clearance and decreases plasma urea and creatinine concentration.
Ethical Considerations
Compliance with ethical guidelines
This study obtained its ethical approval form the Research Ethics Committee of Arak University of Medical Sciences (Code: 90-106-8). All tests on animals were approved by the ethics committee for laboratory animals of this university.
Funding
This study extracted from a research proposal approved by the Arak University of Medical Sciences. We received financial support from the Deputy for Research and Technology of this university.
Authors' contributions
Conceptualization, methodology, validation, and formal analysis: Saeed Hajihashemi; Investigation, resources, analysis and initial draft preparation: Razieh Rajabi and Atefeh Ghiasabadi Farahani.
Conflicts of interest
The authors declare no conflict of interest
Acknowledgements
The authors would like to thank the Deputy for Research and Technology of Arak University of Medical Sciences for their support.
References
- Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014; 7:457-68. [DOI:10.2147/IJNRD.S39747] [PMID] [PMCID]
- Ali BH, Al Za’abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: A mini‐review of recent research. Basic Clin Pharmacol Toxicol. 2011; 109(4):225-32. [DOI:10.1111/j.1742-7843.2011.00728.x] [PMID]
- Randjelovic P, Veljkovic S, Stojiljkovic N, Jankovic-Velickovic L, Sokolovic D, Stoiljkovic M, et al. Salicylic acid attenuates gentamicin-induced nephrotoxicity in rats. Sci World J. 2012; 2012:390613. [DOI:10.1100/2012/390613] [PMID] [PMCID]
- Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007; 223(1):86-98. [DOI:10.1016/j.taap.2007.05.004] [PMID]
- Randjelovic P, Veljkovic S, Stojiljkovic N, Sokolovic D, Ilic I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017; 16:388-99.
- Morshedloo M, Morshedloo M, Pirali Hamedani M, Yazdani D. An over review to Origanum vulgare L. and its pharmacological properties. J Med Plants. 2018; 4(68):15-31.
- Oniga I, Pușcaș C, Silaghi-Dumitrescu R, Olah N-K, Sevastre B, Marica R, et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules. 2018; 23(8):1-14. [DOI:10.3390/molecules23082077] [PMID] [PMCID]
- Mirzaee A, JaberiHafashani H, Madani A. [Antioxidant activities, total phenols and total Flavonoids assay of Origanurm vulgare, Teucrium polium and Thymus daensis (Persian)]. Hormozgan Med J. 2012; 15(4):285-94.
- Sharifi-Rigi A, Heidarian E. Therapeutic potential of Origanum vulgare leaf hydroethanolic extract against renal oxidative stress and nephrotoxicity induced by paraquat in rats. Avicenna J Phytomed. 2019; 9(6):563-73.
- Foroozandeh M, Bigdeli M, Rahnema M. [The effect of hydroalcoholic extract of Origanum vulgare on Blood Brain Barrier (BBB) permeability and neurologic deficits in rat stroke model (Persian)]. J Torbat Heydariyeh Univ Med Sci. 2014; 2(3):1-9.
- Srihari T, Sengottuvelan M, Nalini N. Dose‐dependent effect of oregano (Origanum vulgare L.) on lipid peroxidation and antioxidant status in 1, 2‐dimethylhydrazine‐induced rat colon carcinogenesis. J Pharm Pharmacol. 2008; 60(6):787-94. [DOI:10.1211/jpp.60.6.0015] [PMID]
- Bankova R. Oleum oregano-properties and application. Tradit Modern Veterin Med. 2017; 2(1):25-30.
- Hajihashemi S, Hamidizad Z, Rahbari A, Ghanbari F, Motealeghi ZA. Effects of cobalamin (Vitamin B12) on gentamicin induced nephrotoxicity in rat. Drug Res. 2017; 67(12):710-8. [DOI:10.1055/s-0043-117418] [PMID]
- Ahmadi F, Hajihashemi S, Rahbari A, Ghanbari F. Effects of nitroglycerine on renal ischemia-reperfusion injury in adult male rats. Drug Res. 2019; 69(11):612-20. [DOI:10.1055/a-0958-1987] [PMID]
- Hajihashemi S, Jafarian T, Ahmadi M, Rahbari A, Ghanbari F. Ameliorative effects of zataria multiflora hydro-alcoholic extract on gentamicin induced nephrotoxicity in rats. Drug Res. 2018; 68(07):387-94. [DOI:10.1055/s-0043-124968] [PMID]
- Cuzzocrea S, Thiemermann C, Salvemini D. Potential therapeutic effect of antioxidant therapy in shock and inflammation. Current Med Chemist. 2004; 11(9):1147-62. [DOI:10.2174/0929867043365396] [PMID]
- Savin V, Karniski L, Cuppage F, Hodges G, Chonko A. Effect of gentamicin on isolated glomeruli and proximal tubules of the rabbit. Lab Invest. 1985; 52(1):93-102.
- Aydin G, Gökçimen A, Öncü M, Çicek E, Karahan N, Gökalp O. Histopathologic changes in liver and renal tissues induced by different doses of diclofenac sodium in rats. Turkish J Vet Anim Sci. 2003; 27(5):1131-40.
- Karahan I, Ateşşahin A, Yılmaz S, Çeribaşı A, Sakin F. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005; 215(3):198-204. [DOI:10.1016/j.tox.2005.07.007] [PMID]
- Gowrisri M, Kotagiri S, Vrushabendra Swamy B, Archana Swamy P, Vishwanath K. Anti-oxidant and nephroprotective activities of Cassia occidentalis leaf extract against gentamicin induced nephrotoxicity in rats. Res J Pharm Biol Chem Sci. 2012; 3:684-94.
- Bae WK, Lee J, Park JW, Bae EH, Ma SK, Kim SH, et al. Decreased expression of Na+/K+-ATPase, NHE3, NBC1, AQP1 and OAT in gentamicin-induced nephropathy. The Korean J Physiol Pharmacol. 2008; 12(6):331-6. [DOI:10.4196/kjpp.2008.12.6.331] [PMID] [PMCID]
- Martínez-Salgado C, Eleno N, Tavares P, Rodríguez-Barbero A, García-Criado J, Bolaños JP, et al. Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int. 2002; 62(5):1682-92. [DOI:10.1046/j.1523-1755.2002.00635.x] [PMID]
- Kaurinovic B, Popovic M, Vlaisavljevic S, Trivic S. Antioxidant capacity of Ocimum basilicum L. and Origanum vulgare L. extracts. Molecules. 2011; 16(9):7401-14. [DOI:10.3390/molecules16097401] [PMID] [PMCID]
- Ali Nam, Saeed SZ. Nephro-protective effect of Punica granatum in gentamicin-induced nephrotoxicity in rats. Med J Babylon. 2012; 9(1):220-8.
- Kaledaite R, Bernatoniene J, Majiene D, Dvorackova K, Masteikova R, Muselik J, et al. Investigation of antiradical activity of Salvia officinalis L., Urtica dioica L., and Thymus vulgaris L. extracts as potential candidates for a complex therapeutic preparation. J Med Plants Res. 2011; 5(25):6090-6.
- Williams P, Trimble M, Crespo L, Holohan P, Freedman J, Ross C. Inhibition of renal Na+, K+-adenosine triphosphatase by gentamicin. J Pharmacol Exp Ther. 1984; 231(2):248-53.
- Sohn EJ, Kang DG, Lee HS. Protective effects of glycyrrhizin on gentamicin‐induced acute renal failure in rats. Pharmacol Toxicol. 2003; 93(3):116-22. [DOI:10.1034/j.1600-0773.2003.930302.x] [PMID]
- Neugarten J, Aynedjian HS, Bank N. Role of tubular obstruction in acute renal failure due to gentamicin. Kidney Int. 1983; 24(3):330-5. [DOI:10.1038/ki.1983.162] [PMID]
Type of Study: Original Atricle |
Subject:
Basic Sciences
Received: 2019/08/14 | Accepted: 2019/09/7
Received: 2019/08/14 | Accepted: 2019/09/7
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |